Supplement Packaging
Retail-ready, audit-ready, and built to move. APEX CSM engineers packaging that protects potency, accelerates speed-to-shelf, and clears retailer & marketplace intake with fewer revisions—so you can launch sooner and scale with confidence.
Request a Quote
Built for Speed, Compliance, and Scale
From dielines and flavor panels to tamper evidence and lot coding, we align packaging choices to your channels and budget. FDA- & cGMP-certified facilities, flexible MOQs, and disciplined QA keep schedules realistic and documentation clean.
Fast Lead Times
Vetted converters and stock components minimize waits. We quote realistic dates and manage critical paths across materials and prepress.
Flexible MOQs
Start lean with stock sizes and right-size first runs. Scale into custom molds or finishes as velocity grows.
FDA & cGMP Network
Audit-ready SOPs, lot traceability, and label accuracy workflows safeguard brand trust and intake approvals.
Core Packaging Types
Choose from proven formats optimized for supplement stability, logistics efficiency, and shelf impact. We’ll align closures, seals, and labeling to your formula and channel strategy.
Bottles & Jars
HDPE, PET, and glass with induction seals, desiccants, and child-resistant or snap caps for capsules, tablets, and gummies.
Blister Packs
PVC/PVDC, Aclar®, or aluminum-aluminum barrier systems with printed blister cards and lot coding—ideal for dosing control.
Stick Packs
Single-serve sticks for powders with high-speed fill accuracy, easy-open notches, and cartonizing for retail or DTC.
Sachets & Pouches
Multi-layer films for moisture/oxygen control, resealable zippers, and scoop insertion for proteins and functional blends.
Retail Cartons
Folding cartons and display shippers with gloss/matte finishes, inserts, and transit-ready barcoding.

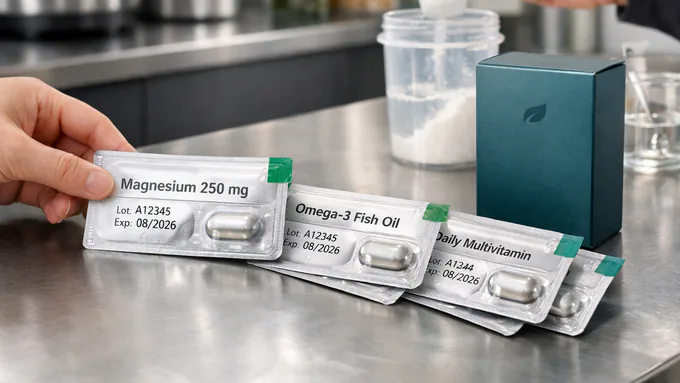
What You Can Expect
- Single accountable partner aligning packaging with formula, claims, and channels.
- Clear artwork & prepress guidance: dielines, bleed, barcodes, and color targets.
- Right-sized first runs leveraging stock components to de-risk cash flow.
- Tamper evidence, induction sealing, and lot traceability standardized.
- Retail & marketplace intake support, including Amazon-ready documentation.
Packaging At-a-Glance
Select from proven formats tuned for stability, speed, and shelf impact.
- Bottles & Jars:HDPE, PET, glass; induction seal + neckband.
- Blisters:PVC/PVDC or Alu-Alu for higher moisture barriers.
- Stick Packs:Single-serve powder, easy-open, retail cartonizing.
- Pouches:Multi-layer films, resealable zippers, scoop insertion.
- Cartons:Folding/SRP, GS1 barcodes, matte or gloss finishes.
Our Packaging Workflow
A predictable path from concept to shelf. Fewer handoffs, fewer surprises.
Brief
Define channels, claims, storage, and budget to select the right format and components.
Specs & Quotes
Confirm materials, closures, finishes, and print method; quote MOQs and lead times.
Artwork & Prepress
Provide dielines and check barcodes, claims, and supplement facts for intake readiness.
Procure & Produce
Source components; run fill/pack under cGMP with in‑process checks and lot coding.
Ship & Handoff
Release with COAs and traceability; retail/DC prep or DTC/3PL handoff as needed.
Packaging FAQs
How do you determine the best packaging for my formula?
We evaluate water activity, sensitivity to light/oxygen, serving size, and channel needs (DTC vs retail) to recommend formats and barriers that protect actives and control cost.
Can you support Amazon and retailer intake?
Yes. We align labels and packaging to marketplace and retailer specs, including FNSKU/GS1 barcodes, warnings, and image‑ready panels to reduce listing delays.
What tamper‑evident features do you offer?
Induction seals, pressure seals, neckbands, and shrink sleeves. We’ll match options to your product and regulatory expectations.
Do you have sustainable packaging options?
We can source PCR resins, recyclable films, and minimized carton footprints. We balance sustainability goals with performance and shelf life.
What are typical lead times?
Stock components move fastest. Custom finishes and printed materials add prepress and conversion time. We quote timing up front and update at checkpoints.