Custom Liquid Supplement Manufacturing
From tinctures and shots to syrups and emulsions, APEX CSM engineers liquids for taste, clarity, and shelf-life—then fills at scale in FDA- and cGMP-certified facilities.

Liquids Built for Taste, Clarity, and Throughput
We balance solubility, preservation, and sensory so your first production fill behaves like your pilot—no surprises at torque, fill volume, or clarity.
Solubility & Emulsion Design
Carrier selection (water, glycerin, ethanol, MCT) and emulsifiers tuned for actives; cloud point and particle size targeted for stability and mouthfeel.
Preservation & Shelf-Life
pH and water activity designed with appropriate preservative systems and packaging barriers to support labeled shelf-life.
Channel-Ready Compliance
COAs, Supplement Facts logic, and packaging specs aligned to FDA and retailer/marketplace requirements to keep listings live.
Liquid Options
- Tinctures, syrups, & elixirs
- Shots & RTD concentrates
- Emulsions & fine suspensions
- Alcohol-free & sugar-free builds
Sensory & Experience
- Flavor systems & maskers
- Sweetness/acid balance & BRIX
- Clarity, color, and aroma
- Viscosity & mouthfeel targets
Quality & Stability
- Identity, potency, purity to spec
- pH, specific gravity, BRIX
- Micro & heavy metal compliance
- Accelerated stability guidance
Packaging
- Glass (amber/flint) & PET/HDPE
- Droppers, CRC, pumps, sprays
- Tamper-evident bands & seals
- Nitrogen headspace (by facility)

Our Liquid Workflow
A five-stage path that protects flavor, stability, and fill accuracy while tightening documentation at each step.
Brief & Targets
Claims, audience, carrier, sweetness/acid targets, and packaging set to a manufacturable plan with costed options.
Architecture
Solubilization/emulsion strategy, preservative system, and pH range established; risks and stability levers flagged early.
Prototype
Lab batches with accept criteria for pH, viscosity, BRIX, clarity, potency, and sensory notes.
Pilot & Fill Verification
Line trial confirms fill volume accuracy, capping torque, seal integrity, and packaging fit; documentation validated.
Release
Final spec, master label, COAs, and production package for repeatable scale and channel onboarding.
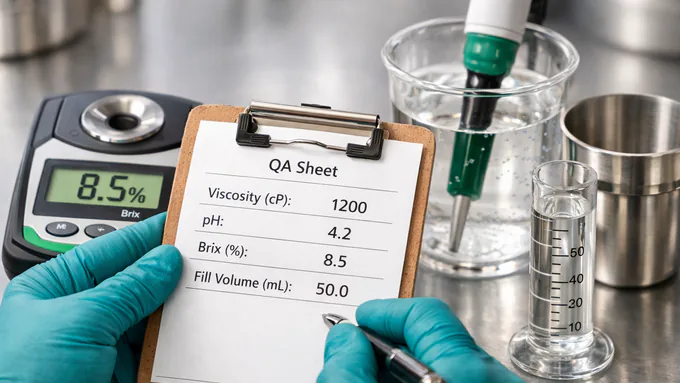
Quality, Documented
Every prototype and lot is traceable. In-process checks protect stability and presentation while keeping audits straightforward.
- pH, viscosity (cP), specific gravity/BRIX
- Potency, microbial, and heavy metals
- Fill volume accuracy & cap torque
- Change control & versioned formulas
Liquid Specialties
Differentiate with carrier, clarity, and closure—without sacrificing throughput or compliance.
Alcohol-Free & Sugar-Free
- Glycerin & water-based builds
- Natural flavors & sweeteners
- Low-calorie claim strategies
- Allergen & free-from options
Emulsions & Suspensions
- Oil-in-water & nano-emulsion builds
- Sedimentation control & redispersibility
- Light & oxygen protection
- Stability rationale & packaging fit
Brand Presentation
- Clarity, color, and flavor profile
- Custom glass & closures (CRC/droppers)
- Tamper-evident & shrink banding
- Retail-ready cartons & kitting
Liquid FAQs
Answers on stability, preservation, packaging, and timelines.
Do you support alcohol-free formulations?
Yes. We build glycerin- and water-based systems with appropriate flavor, sweetness, and preservative strategies to meet your claims and shelf-life goals.
How do you approach preservative systems?
We design around pH, water activity, and packaging barriers, then verify with micro testing aligned to your label and distribution conditions.
What closures and bottles can you supply?
Glass (amber/flint) and PET/HDPE with droppers, child-resistant caps, pumps, and sprays—finished with induction seals and tamper-evident bands.
What are typical lead times and MOQs?
Timelines depend on sourcing, packaging, and formula complexity. We support flexible MOQs and propose the fastest compliant path to launch.